Introduction
Lung cancer remains the deadliest form of human cancer, but in its early stages it can often be cured via surgical tumor resection [1]. In this regard, lung nodules (LNs) are often identified in at-risk patients via low-dose computed tomography (CT) approaches [2–4], after which video-assisted thoracoscopic surgery (VATS)-guided wedge resection can be used to simultaneously treat and diagnose LNs. The minimally invasive VATS procedure enables treated patients to recover rapidly following surgery, but can be challenging to implement in patients with small LNs or ground-glass nodules (GGNs) as it is difficult to palpate these nodules [1]. Sub-centimeter (≤ 1 cm) LNs (SCLNs) are particularly difficult for surgeons and pathologists to accurately treat and diagnose.
The utilization of preoperative CT-guided approaches to LN localization can substantially improve the rates of successful LN detection during VATS, thus reducing the frequency with which patients must undergo conversion from closed to open thoracotomy [5]. Furthermore, some LNs which cannot be palpated usually require VATS lobectomy. However, if the LNs are non-malignant diseases, there is no need to perform lobectomy; wedge resection alone is sufficient [6]. Therefore, preoperative localization for LNs also can decrease the rate of unnecessary lobectomy. A number of materials have been utilized for preoperative LN localization, such as coils, hook-wire, methylene blue, and radio-labeling agents, with coil localization being associated lower complication rates than other strategies [6]. Given its advantageous properties, coil-based localization is commonly used for complex or difficult procedures such as those in patients with sub-fissural, bone-occluded, or multiple LNs [7–9]. Few studies to date, however, have assessed the efficacy of CT-guided coil localization for SCLNs.
Aim
This study was designed to assess the clinical efficacy of preoperative CT-guided coil localization for SCLNs.
Material and methods
The Institutional Review Board of our institution approved the present study. As this study was retrospective in design, written informed consent was not required.
Patients
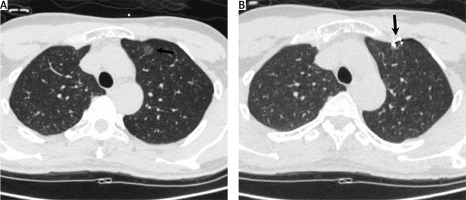
We conducted a retrospective analysis of consecutive patients with SCLNs who underwent CT-guided coil localization followed by VATS at our hospital within January 2015–December 2019. The inclusion criteria for this study were: (a) patients with LNs considered to exhibit an intermediate-to-high cancer risk upon clinical and radiological examination [10], (b) patients with LNs ≤ 1 cm in size (Figure 1 A), and (c) patients with a ≤ 3 cm LN-pleural distance. Patients were excluded from the present study if they met the following criteria: (a) patients exhibiting diffused LNs, (b) patients exhibiting typical benign LNs such as calcifications, (c) patients with LNs that decreased in size during the follow-up period, and (d) patients with coagulatory dysfunction, active infections, active bleeding, or limited cardiopulmonary reserve.
Coils
Individual 50 mm long, 0.038-inch diameter fiber-coated coils (Cook, IN, USA) loaded into cannulas were used for localization procedures.
CT-guided coil localization
Coil localization was guided by 16-slice CT (Philips, OH, USA), with patients under local anesthesia.
Patients were placed at an appropriate position based on LN location, with an appropriate needle pathway being selected based on preoperative CT images. An 18 G coaxial needle (Precisa, Roma, Italy) was used to puncture the lung, after which the tip of the needle was advanced until it was within 1 cm of the LN. The coil was then inserted, with the coil tail remaining visible as described previously [11]. After insertion of the coil into the lung tissue proximal to the LN, the needle sheath was slowly removed so that the coil tail was above the visceral pleura (Figure 1 B).
VATS-guided wedge resection
VATS was conducted within 24 hours of coil localization, with wedge resection being conducted based on coil tail visualization above the visceral pleura, with the resected edge being > 2 cm from the coil. When coil tail visualization was not possible during the VATS procedure, wedge resection was instead performed via palpation of the coil. If neither coil visualization nor palpation was successful, a lobectomy was instead conducted.
Results
Patients
In total, we analyzed 52 patients with SCLNs in the present study. These patients had a mean age of 57.1 ±10.1 years, and 15 of these patients had a history of prior malignancies, including 9 patients with a history of prior lung cancer resection.
Coil localization
In total, 66 LNs were localized in these 52 patients (Table I), with one coil being used per LN. In this cohort, CT-guided coil localization was associated with a 93.9% (62/66) technical success rate. In the remaining 4 patients, this localization procedure failed because the toil was fully inserted into the lung. The technical success rates of coil localization were comparable between solid and sub-solid (mixed GGNs and GGNs) SCLNs (91.2% vs. 96.9%, p = 0.332). CT-guided coil localization procedures lasted a mean of 15.2 ±4.5 minutes.
Table I
Characteristics of the 66 lung nodules
Localization-related complications
Pneumothorax and hemoptysis occurred in 6 (11.5%) and 4 (7.7%) patients, respectively, following coil localization. Of the patients with pneumothorax, one required the insertion of a chest tube to treat this condition.
Wedge resection
VATS-guided wedge resection was associated with a 100% technical success rate in the present patient cohort, with no patients undergoing conversion to thoracotomy. In total, 62 LNs were resected based on coil tail visualization, while the remaining 4 LNs were resected following coil palpation. One-stage VATS-guided wedge resection was conducted in the 12 patients with multiple SCLNs.
All resected SCLNs were successfully identified following coil localization and resection by a trained pathologist. Of these patients, 13 underwent further lobectomy as a consequence of being diagnosed with invasive adenocarcinoma. The VATS procedure was associated with a mean duration of 128.9 ±66.7 minutes and a mean blood loss of 83.0 ±67.7 ml.
Final diagnoses
All 66 SCLNs were diagnosed as being either malignant (n = 38) or benign (n = 28), with sub-solid SCLNs (mixed GGNs and GGNs) more frequently being diagnosed as malignant relative to solid SCLNs (81.3% vs. 32.4%, p < 0.001).
A total of 17 SCLNs were found in 15 patients with previous malignancy. The 17 lesions were primary lung cancer in 10 cases, benignities in 6 cases, and metastasis in 1 case.
Discussion
SCLNs are most commonly detected incidentally in patients undergoing thoracic CT evaluation. These SCLNs are managed through regular CT-based follow-up, biopsy, and surgical resection as appropriate [10]. While follow-up schedules for the management of LNs have been produced by the Fleischner society [3], these schedules are best suited to LNs that are considered to be at low risk of transforming into lung cancer. When LNs are associated with an intermediate-high risk of lung cancer, more invasive diagnostic methods such as biopsy or wedge resection are instead appropriate. CT-guided biopsy is a highly accurate approach to diagnosing SCLNs [12, 13], but it is associated with a ~10% diagnostic failure rate [12], and it remains a relatively difficult technique to conduct.
Surgical resection is the standard reference approach to the definitive diagnosis of SCLNs. When conducting anatomical segmentectomy or wedge resection procedures, preoperative LN localization can markedly improve success rates [14–16]. A range of localization techniques have been tested to date [6, 14–16], with coils, hook-wire, and methylene blue being among the most commonly utilized materials associated with these techniques [6]. Relative to methylene blue- or hook-wire-based localization, coil localization requires a higher level of skill to successfully execute, given that the coil tail must be positioned so as to remain on the visceral pleura. In contrast, methylene blue can rapidly diffuse away from the LN of interest, while a hook-wire can easily become dislodged, resulting in pneumothorax and limiting the utility of these approaches [6].
Herein, we found that CT-guided coil localization and VATS-guided wedge resection were associated with technical success rates of 93.9% and 100%, respectively. These rates are consistent with those from other studies evaluating the preoperative coil localization of LNs [7, 8, 11]. Our 93.9% technical success rate suggests that it is highly feasible to implement CT-guided coil localization in patients with SCLNs. We employed a localization technique wherein the coil tail remains visible, although Su et al. [17] previously reported no significant difference in the clinical efficacy of coil localization strategies in which the coil tail remained visible relative to those in which the entire coil was inserted. We opted for the former approach, as the coil tail can be used to directly guide the VATS-guided wedge resection procedure, with intraoperative palpation of the coil being conducted when the coil tail was completely inserted as discussed previously [17]. As such intraoperative palpation prolongs the duration of the VATS procedure, however, the coil tail should remain visible during localization whenever possible.
A total of 12 patients in the present study presented with multiple LNs, and 9 patients had a history of undergoing prior resection to treat lung cancer. Importantly, VATS-guided wedge resection preserved maximal lung functionality in these patients, with a one-stage resection procedure successfully being conducted in all patients exhibiting multiple LNs. These data thus support the use of coil localization as a reliable means of guiding one-stage VATS wedge resection.
In total, 57.6% of resected SCLNs in this study were ultimately diagnosed as being malignant, consistent with previous reports pertaining to the relative malignancy rates for SCLNs (50–79.6%) [12, 13]. We additionally determined that malignancy rates were higher for sub-solid SCLNs relative to solid SCLNs. This is in line with prior results indicating that a sub-solid LN component is independently associated with the risk of malignancy [18, 19].
There are multiple limitations to the present study. As a single-center retrospective analysis, our results are susceptible to selection bias. Furthermore, our sample size was limited, thus preventing us from drawing definitive conclusions pertaining to this localization strategy. In addition, we did not include a control group or groups that underwent alternative localization techniques. Future randomized controlled trials will therefore be required in order to establish the relative efficacy of this localization strategy.