Hemangioma is a benign vascular neoplasm characterized by capillary proliferation, which is more common in children with a prevalence of 4–5% [1]. Hemangiomas are more likely to be present in the head and neck region (65% of cases) and may occur in any organ of the body, such as the skin, subcutaneous tissue, tongue, brain, and liver, but are extremely rare in the thyroid gland [2]. Most cases of thyroid hemangiomas are secondary to neck procedures such as fine-needle aspiration (FNA) or neck trauma that results from the formation of a hematoma [3].
On the other hand, primary thyroid hemangioma is a developmental anomaly due to the lack of canal formation by angioblastic mesenchyma [4]. These tumors have no obvious clinical manifestations other than cervical mass and no distinct imaging signs are seen with ultrasound or computed tomography (CT) scans. In almost all patients, the diagnosis depends on postoperative histopathology [5]. Here, we report a rare case of thyroid hemangioma in which the retrosternal goiter was suspected preoperatively and review the associated literature.
A 63-year-old man was admitted to the Surgery Department of Ghaem Hospital of Mashhad (Iran) with a history of swelling in the front of the neck for 2 months. The patient had orthopnea and dyspnea on exertion but did not mention other symptoms such as pain over the swelling, hoarseness, dysphagia, or voice change. His past medical history was unremarkable, and also, there was no history of FNA biopsy, neck trauma, ionizing radiation exposure or other intervention in the neck. He had no family history of thyroid disease such as thyroid cancer and was a lifelong non-smoker.
On physical examination, a large goiter was present at the inspection and palpation identified a solid, firm, mobile mass in the thyroid region, which measured about 5 × 6 cm. The patient had no signs of hypo- or hyperthyroidism and lymphadenopathy. Biochemical and hematological tests showed no abnormal values. Thyroid function tests revealed that the patient was euthyroid.
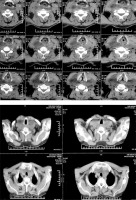
Ultrasonography of the neck revealed that both thyroid lobes were quite large but had no space-occupying lesions. FNA cytology examination was not performed due to patient disagreement. Also, the neck CT scan of the patient is shown in Figure 1. As a result, based on the evidence obtained, the preoperative clinical diagnosis was a retrosternal goiter.
According to the patient’s clinical symptoms, bilateral goiter, and progressive course of the mass, a total thyroidectomy was performed. An intraoperative frozen section was also performed, which an expert pathologist reported as a benign lesion.
Histopathological evaluation of the right lobe showed proliferation of thyroid follicles, most of which contained colloids and cubic cells, surrounded by fibrous tissue and normal thyroid tissue. The histological diagnosis of the right lobe was multinodular goiter. Macroscopic histopathological evaluation of the retrosternal mass revealed a 7 × 3 × 0.5 cm circumscribed hemorrhagic lesion. Microscopic examination showed vascular proliferation with a flat endothelial lining in the context of adipose connective tissue. The final histological diagnosis of the retrosternal mass was cavernous hemangioma.
The patient had no complications after surgery, was followed up for 6 months, and remained asymptomatic.
Hemangioma is a vascular tumor that derives from the endothelial cells. It is more common in children and is generally congenital [6]. Another important point is that hemangiomas are more common in the skin and subcutaneous tissue, and hemangiomas of the thyroid gland are uncommon [2]. Also, thyroid hemangioma is more common secondary to previous neck procedures, so primary thyroid hemangioma is extremely rare and few cases have been reported in the literature. Since our patient did not have a history of FNA or neck trauma, secondary hemangioma was ruled out.
The first case of thyroid hemangioma was reported by Pickleman et al. [7], in 1975, with a mass of 7.5 × 3.0 × 2.0 cm in the left lobe of the thyroid gland. Preoperative diagnosis was made by 99mTc angiography and the mass was surgically removed [7]. After that, a few other cases were reported that are compared in Table I [4, 5, 7–29].
Table I
Characteristics of reported cases of thyroid hemangioma
| First author | Year published | Country | Age [years] | Sex | Size [cm] | Location | Histology |
|---|---|---|---|---|---|---|---|
| Pickleman et al. [7] | 1975 | United States | 56 | Male | 7.5 × 3 × 2 | Left | Cavernous |
| Queiroz et al. [8] | 1978 | Portugal | 29 | Female | 6.5 × 4 × 2.5 | Right | Cavernous |
| 33 | Male | 9 × 7 × 5.5 | Right | Cavernous | |||
| 54 | Male | 9 | Right | Cavernous | |||
| Hernández et al. [9] | 1979 | Spain | 55 | Female | 2 | Right | Capillary |
| Ismaĭlov et al. [10] | 1981 | Russia | 55 | Male | 10 × 6 × 5 | Right | Cavernous |
| Ishida et al. [11] | 1982 | Japan | 46 | Male | 14 × 10 × 9.5 | Right | Cavernous |
| 4 | Male | 1.8 × 0.9 × 0.3 | Isthmus | Cavernous | |||
| 57 | Female | 8 × 6.5 × 3 | Right | Cavernous | |||
| Yokota et al. [12] | 1991 | Japan | 64 | Female | 7.2 × 6 × 4 | Right | Cavernous |
| Pendse and Porwal [13] | 1998 | India | 53 | Male | 6 × 3.5 | Right | Cavernous |
| Kumar et al. [4] | 2000 | India | 53 | Male | 4 × 4 | Right | – |
| Rios et al. [14] | 2001 | Spain | 63 | Female | 5 × 3 | Left | Cavernous |
| 48 | Female | 5 × 4 | Left | Cavernous | |||
| Kumamoto et al. [15] | 2005 | Japan | 56 | Female | 7 × 6 | Right | Cavernous |
| Senthilvel et al. [16] | 2005 | Ireland | 24 | Male | 6 × 5 | Right | – |
| Kano et al. [17] | 2005 | Japan | 21 | Male | 5.5 × 3 × 2 | Right | Cavernous |
| Lee et al. [18] | 2007 | South Korea | 66 | Male | 17 × 16.5 | Left | Cavernous |
| Ciralik et al. [19] | 2008 | Turkey | 64 | Male | 7 × 6 × 6 | Right | Cavernous |
| Datta et al. [20] | 2008 | India | 25 | Male | 4.4 × 4.9 | Left | Cavernous |
| Sakai et al. [21] | 2009 | Japan | 71 | Female | 5.2 × 4.8 × 3.5 | Left | Cavernous |
| Michalopoulos et al. [5] | 2012 | Greece | 78 | Male | 4 × 4 | Right | Cavernous |
| Maciel et al. [22] | 2011 | Brazil | 80 | Female | 22 × 21 × 17 | Left | Cavernous |
| Gutzeit et al. [23] | 2011 | Switzerland | 84 | Female | - | Left | Cavernous |
| Jacobson et al. [24] | 2014 | Canada | 3 M | Female | 3.1 × 3.7 × 2.2 | Right | - |
| Dasgupta et al. [25] | 2014 | India | 38 | Male | 6 × 5 × 3 | Left | Cavernous |
| Miao et al. [26] | 2017 | China | 48 | Male | 4 × 3.5 | Right | Cavernous |
| Liang et al. [27] | 2020 | China | 2M | Female | 3 × 3 × 1 | Both sides | Capillary |
| Yang et al. [28] | 2020 | China | 24 | Female | 3.6 × 1.2 × 1.5 | Left | – |
| Bains et al. [29] | 2020 | Australia | 77 | Female | 6 × 5.5 × 5 | Right | Cavernous |
Patients usually present with swelling or a mass in the front of the neck. The growth rate of this mass has been reported very differently so that in one study, it lasted 16 years [20], and another was 20 days after the onset of swelling [27]. In our case, it took two months from the onset of swelling to the onset of symptoms. This gradual increase in mass growth may cause compressive symptoms, such as hoarseness, dyspnea, vocal cord paralysis, dysphagia, voice change, and tracheal deviation [7, 18], as our patient had orthopnea and dyspnea on exertion.
Histologically, thyroid hemangiomas are classified as cavernous, synovial, arteriovenous, venous, capillary, and racemose [17]. As in our case, cavernous hemangioma is the most common, followed by capillary hemangioma (Table I).
Maciel et al. [22] studied thyroid hemangioma size; the largest had weight 2800 g, size 22 × 21 × 17 cm, and generally, the diameter varied between 2 and 22 cm. As illustrated in Table I, men were more affected than women, and patients’ age ranged from 2 months to 84 years. Also, in most cases, the tumor location was on either the right or left side, while in the study of Liang et al. [27], it was reported on both sides and Ishida et al. [11] reported location on the thyroid isthmus.
It is challenging to diagnose thyroid hemangioma before surgery because there are no clinical features or pathognomonic findings on ultrasonography, CT scans or FNA [21]. Although FNA biopsy is essential to diagnose thyroid masses in most cases, it will be inconclusive in hemangiomas because only blood is present in the specimens [21]. However, it is crucial to identify thyroid hemangiomas before surgery because they may lead to significant hemorrhage during surgery. Pickleman et al. [7] reported that the patient lost more than 2 l of blood during left hemithyroidectomy and received four units of whole blood. However, our patient underwent a complete thyroidectomy, but all the thyroid vessels were carefully ligated and the mass was removed without rupture, resulting in minor blood loss.
Shpitzer et al. [30] recommended the use of digital subtraction angiography (DSA), magnetic resonance imaging (MRI), red blood cell (RBC) scans, and single-photon emission computed tomography (SPECT) for preoperative diagnosis of thyroid hemangioma. Also, Kumar et al. [4] suggested a technetium-99 erythrocyte-labeled scan to confirm hemangioma. These investigations were not conducted in our case due to high cost and unavailability. Currently, as in our case, almost all patients have been diagnosed by histopathological evaluation of the excised mass [25].
The treatment of choice for thyroid hemangioma is surgical removal by hemithyroidectomy or total thyroidectomy [26]. Our patient also underwent a total thyroidectomy due to compressive symptoms and retrosternal extension.
In conclusion, our case report and literature review indicated that primary thyroid hemangioma is a rare benign disease, mostly escaping preoperative diagnosis. A definitive diagnosis can be made based on histopathological findings. The treatment of choice for thyroid hemangioma is surgery, which has a good prognosis.