Introduction
Cancer is an abnormal growth of cells, which tends to proliferate in an uncontrolled way and sometimes metastasise (spread). Cancer is not one specific type of disease. It is in fact over 100 different types of distinctive diseases. It can be in any tissue of the body and have many forms in each area in the body [1]. It is a group of disorders distinguished by abnormal cell growth and the propensity to infiltrate or spread to other parts of the body. Benign tumours are not cancerous and do not spread to other parts of the body [2]. A lump, unusual bleeding, a long cough, unexplained weight loss, and a change in bowel movement are all possible signs and symptoms. While these symptom might indicate cancer, they can also be caused by other circumstances [3]. Breast cancer (BC) is a potentially fatal tumour that develops from aberrant cells. It has an effect on the tissues involved in the production of milk (ductal and lobular tissues). It has been the most prevalent among women in the preceding 40 years The incidence of BC has remained high in women over the age of 50 years, while the rate of survival has been lower in those under the age of 50 years [4].
Breast cancers can begin in any area of the breast, although the majority of breast tumours develop from cells in the milk-transporting ducts, called ductal malignancies. Some also begin in the milk-producing glands (lobular cancers) [5].
Breast cancer is the greatest common tumour among females, and it represents a major global health problem. Almost 70% of females with BC are aged over 50 years, and only 5% are younger than 40 years old. Approximately 700,000 cases are reported annually worldwide [6]. Breast cancer is the most prevalent cause of malignancy in women globally, with 316,700 cases recorded in 2019 and 41,760 deaths in the United States alone [7]. Cancer is one of the main causes of mortality worldwide [8]. The exact causes of BC are not clearly defined, and it is not just one cause but rather the result of a combination of several factors. These factors lead to an increased risk of developing cancer, such as age, menstruation, alcohol consumption, hormonal factors, lack of breast feeding, environmental factors, genetic factors, obesity, and others [9].
Serine biosynthesis is an essential branch of glycolysis that cancer cells exploit to sustain their energy supply [10]. 3-phosphoglycerate (3-PG), a glycolysis intermediate product, is a precursor to serine production. Serine synthesis pathway (SSP) activity has a function in tumour progression. Extracellular serine is required for cell proliferation; however, when the serine production pathway is dormant, excessive extracellular serine cannot sustain cell proliferation [11]. Phosphoglycerate dehydrogenase (PHGDH) is a critical enzyme in serine biosynthesis that catalyses the first step downstream of glycolysis in the serine biosynthetic pathway [12]. It catalyses the glycolytic intermediary 3-PG to 3-phosphohydroxypyruvate (3-PHP) conversion [13]. Carbon is transferred from the glycolytic route to the SSP in PHGDH-amplified tumour cells, where it may trigger a variety of biosynthetic processes that promote tumour cell growth and proliferation [14]. Phosphoglycerate dehydrogenase, a metabolic enzyme involved in the serine synthesis route, appears to play an important role in cancer development and proliferation. Phosphoglycerate dehydrogenase is a dehydrogenase whose expression in cancer was first discovered in 2010. Because its silencing allows for a considerable decrease in tumour growth, it appears to be a viable target for the development of novel anti-cancer agents [15].
Glycine, the second product of the serine hydroxymethyl transferase process, may be employed directly in the biosynthesis of purine, which contains 2 carbon atoms and one nitrogen atom. Because glycine is a component of glutathione, the cell’s principal antioxidant molecule, it is also required to maintain cellular redox equilibrium [16]. Glycine also feeds heme production in the mitochondria, sustaining oxidative phosphorylation. It has recently been demonstrated that glycine absorption and catalogue list can promote tumour formation and malignancy, implying that glycine metabolism might be a therapeutic target [17]. A sequence of enzymatic activities produces serine and glycine from the glycolysis intermediate 3-PG. Glycine’s methyl group may be utilised to fuel a one-carbon metabolic rate in the folate cycle, which cancer cells use to produce protein, nucleic acids, lipids, and cofactors. Serine and glycine are also involved in the formation of antioxidants, which helps cancer cells survive in hypoxic settings. Glutamate is a by-product of serine synthesis that can be convert to α-ketoglutarate to energise the tricarboxylic acid cycle [18]. Glycine/one-carbon metabolism is also vital in cellular redox equilibrium. For each step of the folate cycle, tetrahydrofolate reductase creates one molecule of NADP+. The modification of the NADP+/NADPH ratio leads to the preservation of a redox state [19]. Inhibition of the component of the folate cycle can reduce growth and proliferation of malignant cells and increased DNA damage [20]. The current study aimed to investigate the levels of PGDH and glycine, the intermediate of serine pathways association with pathogenesis of BC development.
Material and methods
Subject
The total number of samples collected was 135 – 65 samples were collected from women with breast cancer, whose ages ranged from 28 to 53 years, and their mean age was 43.23 ±7.028 years. The samples were collected in the oncology hospital. The patients with BC were subdivided into groups depending on their treatment: patients with chemotherapy (n = 37) and patients with hormonal therapy (n = 28). The study also included 70 samples from healthy women as a control group; their ages ranged from 27 to 45 years, their mean age was 40.86 ±7.948 years, and the sampling period was December 2022 – March 2023. General data were recorded from the patients, including age, gender, family history, and some tests that were conducted such as a urea test and creatine test. All laboratory tests were carried out in the Oncology Hospital Laboratory and the Clinical Biochemistry Laboratory at the University of Al-Qadisiyah Iraq College of Medicine.
Methods
Blood samples (5 ml) were obtained from each member of the study group. 2 ml of blood was placed into an EDTA Vacationer tube. The complete blood count (CBC), neutrophil/lymphocyte, and haemoglobin ratio analysis was done on a CBC haematology analyser. Serum was extracted from the remaining (3 ml) blood for 30 min by centrifugation at 4000 rpm for 15–20 minutes at room temperature 4°C. The separate serum was preserved use Eppendorf tubes (1.5 ml) at –80°C for biochemical analysis of blood urea, nitrogen, and serum creatinine by colorimetric method. Enzyme-linked immunosorbent assay was used for PHGDH and glycine measurements.
Statistical analysis
SPSS 23 was used to analyse the data. The data were expressed as mean ± the standard errors of the mean described. The Andersen-Darling test was used to check for normality. To investigate significant differences between the control and patient groups, Student’s t-test was performed. To test if there were any statistically significant differences between the 3 groups, a one-way ANOVA was employed. A p-value of ≤ 0.05 was considered significant throughout.
Results
Assessment of mean age between patients with breast cancer and control group
The mean ages of individuals with breast tumours and the healthy group are shown in Table 1. The mean ages of the patients and the control subjects were 43.23 ±7.028 years and 40.86 ±7.948 years, respectively; non-significant changes were observed in the mean ages of both groups (p = 0.0951). Family history, breast type, and location are summarised in Table 1.
Table 1
Clinicopathological data and mean age in individuals with breast cancer and control subjects
Haematological characteristics assessment
The haematological features of people with BC and the healthy group are presented in Table 2. The neutrophil/lymphocyte ratio (NLR) was estimated in the current study as a measure of inflammatory response in patients with BC (Table 2). WBC count in the BC group was significantly lower than in the control group (p < 0.05).
Table 2
Comparison of the haematological parameters in study groups
A substantially higher neutrophil count was also seen in cancer patients compared to controls (p < 0.05) (Table 2).
Significant changes in lymphocyte counts (p < 0.05) were found in the results. In addition, the data showed that NLR was considerably higher in the BC group compared to the control group (p < 0.05) (Table 2).
Serum phosphoglycerate dehydrogenase levels in patients with breast cancer and the control group
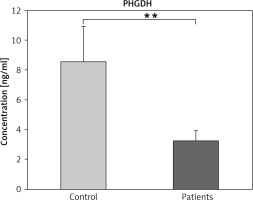
Phosphoglycerate dehydrogenase is a key enzyme that catalysed committed step in L-serine biosynthesis. It is as well as in cysteine and glycine synthesis. The results of current research show a decrease in PHGDH levels in patients with BC compared to controls (p ≤ 0.01) (Fig. 1).
Fig. 1
Serum phosphoglycerate dehydrogenase levels in patient with breast cancer and the control group
The data are presented as mean ±SD.
** Indicates a significant change among patients and controls (p ≤ 0.01)
PHGDH – phosphoglycerate dehydrogenase
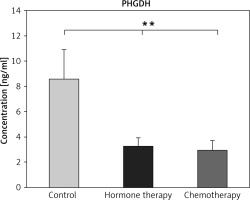
The decrease in PHGDH was clearly observed in patients with chemotherapy compared to controls (p ≤ 0.01) (Fig. 2), but not significantly changed compared to patients treated with hormone therapy (p ≤ 0.01) (Fig. 2).
Serum glycine levels in patients with breast cancer and the control group
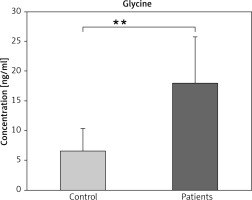
The results showed that the serum glycine level was significantly increased in the patient group with BC compare to the control group (p ≤ 0.01) (Fig. 3).
Fig. 3
Serum glycine levels in patients with breast cancer and controls
The data are presented as mean ±SD.
** Indicated a significant change among patients and controls (p ≤ 0.01)
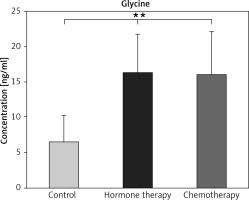
Significantly higher levels of glycine were found in both the hormone and chemotherapy groups compared to controls; however, glycine levels were not changed between the patients who received hormone and those who received chemotherapy (p ≥ 0.01) (Fig. 4).
Discussion
Demographic characteristics of patients and control subjects
The current study found no significant differences in the mean age of BC patients compared to controls. More than 40% of afflicted patients are above the age of 65 years, and this group accounts for over 60% of all BC deaths [21, 22]. Interestingly, the predicted probability of developing BC before the age of 49 years is 1/53; however, this increases to 1/43 for those aged 50–59 years, and again to 1/23 for those aged 60–69 years. Significantly, women over the age of 70 years had the highest risk, with a 1/15 chance of developing breast cancer.
WBC, neutrophil, lymphocyte count, and neutrophil/lymphocyte ratio
The current study found a significant decrease in WBC count in the BC patient group compared to controls (p ≤ 0.05).
In addition, this research demonstrated the existence of a significant increase in neutrophil count in patients with breast cancer compared to controls (p ≤ 0.05) (Table 2). This study agrees with the study by Tripathi et al. [22]. Neutrophils are the most common leucocyte in blood and are advised as the first line of defence. Neutrophils secrete proteinases into the surrounding tissue, which causes destruction to the host cell. Neutrophils are also able to release a variety of cytokines and chemokines, which can affect the inflammatory response, along with the immunological response. The research results showed a significant decline in lymphocyte count (p < 0.05). A low absolute lymphocyte count has been correlated with inferior outcomes in different cancers, such as breast cancer. This study also revealed that the lymphocyte count was significantly decreased in the BC group as compared to the control group [23].
Also, the results indicate that NLR is significantly higher in the patient group compared to control the group (p < 0.05), in agreement with Yoon et al. [23]. This study revealed that the NLR ratio was significantly increased in the BC group compared to the control group. This study suggested that BC risk increased with increasing NLR ratio. Higher NLR is associated with poor survival in patients diagnosed with different types of cancer.
Serum phosphoglycerate dehydrogenase in breast cancer patients
Phosphoglycerate dehydrogenase is the first enzyme and the rate-limit enzyme in serine production pathways. It is highly elevated in several cancers, including BC [25].
Phosphoglycerate dehydrogenase converts 3-PG, a glycolytic intermediate, into 3-phosphohydroxypyruvate. Subsequent enzymes in the SSP, for example PSAT1 and PSPH, then transaminase, and phosphate ester hydrolyses 3-PHP to serine. De novo-synthesis serine and exogenous serine absorbed from the environment are subsequently employed in a variety of biosynthetic processes, such as one-carbon metabolic to support nucleotide synthesis, methylation reactions, and antioxidant defence [26].
Phosphoglycerate dehydrogenase knockdown or inhibitor markedly restrains the growth of these PHGDH-overexpressing cancers, suggest that PHGDH is a promising target for cancer therapy [27].
The results of the study showed a decrease in PHGDH levels in patients with BC compared to controls (p ≤ 0.01) (Fig. 1). These results are consistent with the study by Karaosmanoglu et al., who provided evidence that decreased GPD1 level acted as a diagnostic biomarker in distinguishing triple-negative BC patients from other subtypes [28]. Phosphoglycerate dehydrogenase deficiency can result in cellular DNA methylation loss. Because the methylene unit delivered to the folate cycle by the conversion of serine to glycine can transfer into the methionine cycle, serine indirectly aids in the recycling of homocysteine to methionine as well as the creation of precursors for S-adenosylmethionine. S-adenosylmethionine is a methyl-group donor that is frequently utilised in the methylation of DNA [29]. The previous study showed that inhibition of PHGDH expression blocked the proliferation of PHGDH- expressing tumour cells, suggesting that it could be used as a target for cancer therapy. These findings suggest that PHGDH deficiency makes BC cells more susceptible to chemotherapy and prevents chemotherapy- induced BC enrichment [30].
Phosphoglycerate dehydrogenase knockdown made BC more sensitive to chemotherapy by increasing mitochondrial reactive oxygen species, increasing apoptosis, and decreasing chemotherapy-induced BC enrichment. These findings imply that combining chemotherapy with a PHGDH inhibitor may increase the survival of women with advanced BC by preventing the induction of BC stem cells in response to chemotherapy [17].
Serum glycine in breast cancer patients
Glycine, the simplest amino acid, is obtainable by hydrolysis of protein. Glycine is one of several so-called nonessential amino acids that can synthesise from the amino acids serine and threonine and from other sources and do not require dietary sources. Serine and glycine are biosynthetically linked, and together they provide the necessary precursors for the synthesis of protein, nucleic acids, and lipid that are crucial to tumour cell growth. Moreover, glycine biosynthesis also affects cellular antioxidative capacity, thus supporting tumour homeostasis [25]. This amino acid, which serves as the cell’s primary antioxidant molecule, is also essential to maintain cellular redox homeostasis. Glycine also feeds heme production in the mitochondria, sustaining oxidative phosphorylation [18]. The rapidly grow cancer cell appeared to need glycine for synthesis of purine nucleotide necessary for continued synthesis of DNA. Interfering with glycine metabolism slows growth of the rapidly proliferating cancer cells [31].
The current study showed that blood glycine levels were considerably higher in the BC patient group compared to the control group (p < 0.01) (Fig. 3). Giskeødegård et al. [32] concurred with this study. This increase might be explained by the cellular biosynthesis potential and oxidation caused by using folate derivatives in the one-carbon metabolic rate network due to the cellular biosynthesis potential and oxidation and epigenetic status [33, 34]. Glycine is an amino acid that plays a role in protein, nucleotide, and glutathione production. Glycine’s potential usefulness as a tumour biomarker has also been investigated in human breast cancer, where it was shown to positively correlate with tumour grade. Furthermore, the synthesis of glycine from glucose has been linked to fast cancer cell growth. Surprisingly, higher levels of glycine were linked to HER-2 overexpression; these findings suggest that glycine is a potential biomarker for BC prognosis [16].
The results highlighted that the glycine pathway is critical for BC and indicated that glycine and PHGDH protein levels may serve as prognostic tumour markers in breast cancer.
A better understanding of the serine/glycine metabolic pathway of cancer cells and the influence of this pathway in the tumour microenvironment may provide the rationale for the optimal serine/glycine metabolism-targeted therapies.
Conclusions
Decreased PHGDH and increased glycine levels in patients with BC are associated with rapid growth of cancer cells that need glycine for synthesis of purine nucleotide, suggesting that these intermediate might have a novel function independently of their role in metabolism and might be proposed as non-invasive predictive biomarkers for BC.