Introduction
Acute kidney injury (AKI) is one of the most common problems after cardiac surgery, which increases risk of death [1]. AKI is usually found after aortic surgery more than isolated coronary artery bypass surgery or valve surgery [2, 3].
Increase of serum creatinine may limit early or accurate detection of AKI. Novel biomarkers have been developed that are more sensitive and accurate for clinical use such as neutrophil gelatinase-associated lipocalin (NGAL), liver-type fatty acid binding protein (L-FABP) or tissue inhibitor of metalloproteinase-2 with insulin-like growth factor-binding protein 7 (IGFBP7) in urine.
Aim
This study aimed to evaluate the incidence and risk factors of AKI after hypothermic circulatory arrest (HCA) in aortic surgery patients. We chose urine NGAL as one of the surrogate markers of kidney injury due to accessibility and reliability of this novel biomarker. We hypothesized that urine NGAL can predict AKI with more sensitivity after aortic surgery.
Material and methods
We designed a prospective observational study in patients who underwent aortic surgery that required the HCA technique in King Chulalongkorn Memorial Hospital (KCMH) between May 2019 and August 2021. The inclusion criteria were age above 18 years and lower body temperature under 28 °C measured by rectal temperature. Exclusion criteria were end stage renal disease patients who received chronic renal replacement therapy such as regular hemodialysis or peritoneal dialysis, and previous renal transplantation. We also excluded patient who died within 24 hours after the operation or patients who were unable to collect a urine sample. The technique of cannulation and extent of the operation depended on aortic disease and surgeon preferences.
Operative techniques and hypothermic approach
Our operative technique depended on surgeon preferences and extent of disease. Most of the patients have been diagnosed with acute type A dissection or intramural hematoma type A. For patients without aortic arch intervention we usually used arterial cannulation at the femoral artery, venous cannulation at the right atrium, followed by cooling down until the body temperature at the nasal and rectal probes reached the expected temperatures. For moderately hypothermic (20–25°C) patients we usually used right axillary artery end-to-side suture with an 8-mm Dacron graft as arterial cannulation combined with antegrade cerebral perfusion cannulation into other arch vessels. Cardioplegia depended on surgeon preference such as blood cardioplegia, Custodiol or modified Del Nido cardioplegia.
We collected patients’ blood and urine for analysis at pre-operative (less than 24 hours before operation), immediate post-operative, post-operative 6-hour/24-hour/ 48-hour and post-operative day 7 time points. Blood specimens were sent immediately for laboratory testing. For urine, we kept it in a refrigerator at under 4°C and sent it to the laboratory within 24 hours to store under –80 °C. This technique can keep urine specimens for 1 year [4].
Acute kidney injury (AKI) status was defined by KDIGO (Kidney Disease: Improving Global Outcomes) 2012 criteria. We used the KDIGO criteria to define AKI in our population. AKI was classified into 3 stages: 1, 2 ,3.
Statistical analysis
SPSS 22.0 for Windows (IBM Corp., USA) was used to perform statistical analysis. Qualitative variables are reported as frequency and percentage. Qualitative variables are reported as median and interquartile range (IQR) or mean with standard deviation (SD). For continuous variables, we used Student’s t-test or non-parametric Mann-Whitney U test. For categorical variables, we used the χ2 test or Fisher’s exact test. For analysis of urine NGAL for predicting post-operative AKI, we calculated the area under the receiver-operating characteristic curve (AUC). For other risk factors of post-operative AKI, we used multivariate logistic regression analysis. P-values < 0.05 were considered significant.
Results
Pre-operative and operative data
A total of 58 patients were recruited. The median age was 58 years. About half of the patients were female (55.2%, 32 female patients). The main primary diagnosis was acute type A aortic dissection (35 patients, 60.3%). There were 37 patients who underwent deep HCA (DHCA) and 21 patients who underwent moderate HCA (MHCA). Baseline characteristics and comorbidity including malperfusion were not statistically significant different between the two groups (Table I). There was 1 patient in the MHCA group who had pre-operative shock.
Table I
Pre-operative patient baseline characteristics
[i] DHCA – deep hypothermic circulatory arrest, MHCA – moderate hypothermic circulatory arrest, CKD – chronic kidney disease, defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 square meters, persisting for 3 months or more. Categorical data were compared using χ2 or Fisher’s exact test. Continuous data were compared using Wilcoxon rank sum test.
Types of operation were divided into 4 groups: ascending aortic replacement (11 patients), hemiarch replacement (31 patients), extended arch replacement (14 patients) and total arch replacement (2 patients). There were 45 patients who underwent an emergency operation and 13 patients who underwent elective surgery. Median rectal temperature, DHCA started at 17.8 (17.4–18.2)°C vs. MHCA 24.3 (23.8–25.2)°C. Lower body circulatory arrest time was equally around 31 minutes in both groups (Table II).
Table II
Operation data for patient and post-operative clinical outcomes
[i] DHCA – deep hypothermic circulatory arrest, MHCA – moderate hypothermic circulatory arrest, Op time – operative time, CPB time – cardiopulmonary bypass time, ACC time – aortic cross-clamp time, PRC – packed red cells, AF – atrial fibrillation, RRT – renal replacement therapy, DC – discharge. Categorical data were compared using χ2 or Fisher’s exact test. Continuous data were compared using Wilcoxon rank sum test.
The cardiopulmonary bypass (CPB) time was similar between DHCA and MHCA groups (217 vs. 203 minutes, p = 0.74). Aortic cross clamp time was also similar between DHCA and MHCA groups (101 vs. 105 minutes, p = 0.43). The median operative time was the same in DHCA and MHCA groups (373 vs. 372 minutes, p = 0.3). Intraoperative blood transfusion was not statistically significant between the two groups (Table II).
Operative morbidity and mortality
Post-operative bleeding was found in 12 (20.7%) patients: 7 (18.9%) patients in the DHCA group and 5 (23.8%) patients in the MHCA group, which was not a statistically significant difference (p = 0.66). Post-operative neurological outcomes were non-significantly different between groups, favoring MHCA (4.8% in MHCA vs. 10.8% in DHCA, p = 0.43). Other postoperative complications including atrial fibrillation, delirium, pneumonia, ventilator duration, ICU length of stay (LOS), and hospital LOS did not show statistically significant differences between the two groups (p > 0.05; Table II).
Overall, in-hospital mortality was 5.2% (3/58), including 2 deaths in the DHCA (5.4%) and 1 death in the MHCA (4.8%) group, respectively, which was not a statistically significant difference (p = 0.92). Causes of death included acute mesenteric ischemia and multiorgan failure.
Post-operative renal function and serum creatinine and NGAL level
Based on KDIGO criteria, the overall incidence of AKI was 65.6% (N = 38) including 24 (41.4%) patients in AKI stage 1, 7 (12.1%) patients in AKI stage 2 and 7 (12.1%) patients in AKI stage 3. There were 2 patients who needed renal replacement therapy (RRT), one in each group. However, there was no significant difference in the incidence of any AKI or need of RRT between the DHCA and MHCA groups (p = 0.53) (Table II).
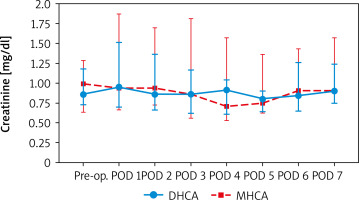
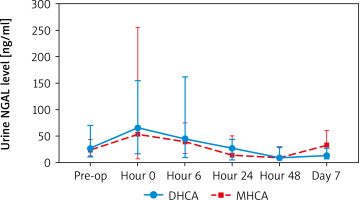
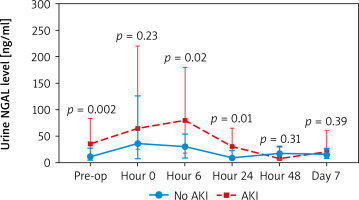
When comparing creatinine and urine NGAL levels from preoperative to postoperative day 7, there were no statistically significant differences between the DHCA and MHCA groups (Tables III, IV, Figures 1, 2). However, when we analyzed AKI and non-AKI groups, there were significantly different urine NGAL levels at pre-operative (p = 0.002), post-operative hour 6 (p = 0.02) and postoperative hour 24 (p = 0.01) (Table V, Figure 3).
Table III
Comparing serum creatinine (mg/dl) at each time point between DHCA and MHCA groups
Table IV
Comparing urine NGAL level (ng/ml) at each time point between DHCA and MHCA groups
Table V
Comparison of urine NGAL level (ng/ml) at each time point between non-AKI and AKI groups
For urine NGAL levels to predict AKI, we found that the urine NGAL level at postoperative hour 0 and hour 6 can predict AKI. At post-operative hour 0, NGAL level more than 20 ng/ml was statistically significantly associated with AKI (odds ratio (OR) = 4.43, confidence interval (CI): 1.33-14.71, p = 0.02) and positive predictive value (PPV) = 75.6. At post-operative hour 6, NGAL level more than 70 ng/ml was statistically significantly associated with AKI (OR = 10, 95% CI: 2.03–49.22, p = 0.005) and PPV = 90.9 (Table VI).
Table VI
Performance of urine NGAL level to predict AKI
Risk factors for AKI
In univariate analysis, risk factors associated with AKI included operative time more than 360 minutes, CPB time more than 200 minutes, and aortic cross clamp (ACC) time more than 100 minutes (Table VII). Advancing age, sex, increasing BMI, LV ejection fraction, hypertension, dyslipidemia, and history of stroke were not statistically significant risk factors of AKI (p > 0.05). Moreover, there was no significant difference between degree of HCA and incidence of AKI (DHCA vs. MHCA, p = 0.66) (Table VII). In multivariable regression analysis adjusted for AKI, only operative time more than 360 minutes was associated with AKI (OR = 19.07, 95% CI: 3.47–104.86, p < 0.001) (Table VII).
Table VII
Risk factors associated with AKI
Discussion
Hypothermic circulatory arrest (HCA) is a necessary method for repair of the proximal aorta and aortic arch surgery. Acute kidney injury (AKI) is a common cause of morbidity after aortic arch surgery, prolonging both ICU and hospital LOS [5–9]. The impact of HCA temperature on post-operative AKI has been evaluated in many studies. Some studies reported that risk factors associated with AKI included CPB time, pre- and intra-operative anemia [10, 11]. The purpose of this study was to determine the incidence and severity of AKI and assess the role of urine NGAL to determine risk of AKI after aortic surgery in our institute.
Our study demonstrated that the overall incidence of AKI after aortic arch surgery was 65.6%, of which only 12.1% was defined as AKI stage 3. In a study from Beijing, China, Cao et al. [12] found that MHCA compared with DHCA reduces the incidence of renal failure (OR = 0.76, 95% CI: 0.61–0.94; p = 0.011) and need for renal replacement therapy. However, our results were similar to other studies from Duke University [5]; the degree of hypothermia was not associated with either incidence or severity of postoperative AKI [5]. Leshnower et al. also reported that moderate degrees of hypothermia with circulatory arrest for aortic arch surgery were not a significant risk factor for renal failure [13].
The optimal degree of hypothermia for cerebral and visceral organ protection during HCA remains a hot issue nowadays. Multiple recent studies have suggested that moderate temperatures 20–28°C (MHCA) may provide equivalent safety while decreasing post-operative bleeding and blood transfusion [14]. Many studies have found no significant difference for temporary neurological deficits between DHCA and MHCA + SACP [12]. Our study results were similar to previous studies in both neurological outcomes and other comorbidities (arrhythmia, bleeding, pneumonia, ICU and hospital LOS). In addition, in hospital mortality was equivalent, 4.8% in MHCA vs. 5.4% in DHCA, which is better than the results from Gong et al. [15], who found that that operative mortality was 10.2% in MHCA and 14.3% in the DHCA group, which did not differ significantly (p = 0.86). The leading cause of death was multi-organ failure, as in our study.
AKI may not present immediately after surgery, and more sensitive options for monitoring AKI are being developed using novel biomarkers. We expected that urine NGAL can predict AKI more sensitively than creatinine level. There has been no previous study on urine NGAL and post-aortic surgery AKI. Ours is the first such study, and we found that urine NGAL immediately post-operatively and at hour 6 in the ICU can predict AKI. Risk factors associated with post-operative AKI included prolonged operative time more than 360 minutes (OR = 19.07, p < 0.001).
One previous study from Cincinnati Children’s Hospital [16], which was a prospective study in children undergoing cardiopulmonary bypass, showed that both urine and plasma NGAL (at a cut-off value of 50 μg/l) levels were powerful independent predictors of AKI. At hour 2 NGAL concentration was a strong independent predictor of clinical outcomes such as duration of AKI. Our study results were similar to other studies [16, 17] in cardiac surgery, but we suggest that a cut-off value of 70 ng/dl at hour 6 is highly associated with AKI after aortic surgery with HCA.
Limitations
There were some limitations of our study. First, it was not a randomized controlled trial study and included quite a small number of subjects when compared to previous studies [5, 15, 18, 19]. Second, the study groups were small, which may have affected the results. Third, we prospectively enrolled patients who underwent mostly hemiarch replacement under HCA, which is around 30 minutes lower body circulatory arrest time. So, our results in both groups may not be statistically significantly different. Therefore, data in this study may not be applicable to patients who underwent more extensive aortic arch surgery that needs longer lower body ischemic time.
Conclusions
AKI after aortic surgery is common. In our population the incidence was greater than 60%. Operative time of more than 360 minutes was a risk factor for AKI. Urine NGAL in the early postoperative period (6 hours) can predict AKI. MHCA is associated with similar post-operative mortality and morbidity outcomes compared to DHCA.