Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease affecting approximately 1% of the global population with high morbidity and mortality [1]. The disease initially presents as an inflammatory response followed by autoantibody activation and synovial and joint damage. Inflammatory activation triggers a series of immune responses. In patients with RA, there is overproduction of autoantibodies, specifically rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA) [2, 3]. When certain peptides in the serum undergo post-translational modifications such as citrullination due to environmental triggers, these altered peptides are recognized as foreign antigens by immune cells, including T-cells. This recognition triggers an immune response, leading to the production of antibodies, such as ACPA [4, 5]. Therefore, RF and ACPA, which represent inflammatory and immune responses in RA, are diagnostic serum biomarkers. However, a subset of RA patients is found to exhibit negative RF and ACPA in serology-related tests, i.e. seronegative rheumatoid arthritis (SNRA) [6, 7]. Since there is no specific point of differentiation in symptoms between SNRA and inflammatory joint diseases such as osteoarthritis, there is still a need to discover new markers for the diagnosis or differential diagnosis of RA and SNRA.
In recent studies, researchers have discovered that levels of interleukin (IL)-9, a cytokine belonging to the common γ-chain family [8], are elevated in both the affected joints and circulation of patients with RA [9-11]. Interleukin 9 exerts its effects by binding to a receptor that consists of an IL-9 receptor α-chain and a γ-chain. This cytokine is primarily secreted by a specific subset of T-helper cells known as Th9 cells. However, Th17 cells, which are involved in inflammation and bone erosion in RA, can also produce IL-9. Interestingly, the frequencies of Th9 and Th17 cells have a strong correlation with the disease activity in RA [11, 12]. Additionally, IL-9 has been shown to enhance the survival of neutrophils and promote the functions of inflammatory T cells [11], further suggesting its involvement in the development of RA. Furthermore, IL-9 has been found to enhance the survival of neutrophils and promote the functions of inflammatory T cells, further implicating its role in the pathogenesis of RA. The above studies suggest that IL-9 could be a new marker for diagnosing RA and a good therapeutic target for future clinical interventions. However, studies of IL-9 in SNRA have not been reported and its diagnostic value for SNRA needs to be further evaluated.
Therefore, in this study, we attempted to screen a new biomarker to distinguish SNRA patients from healthy controls by comparing the levels of different biomarkers. Further, by constructing a rat model of RA, we explored the potential role of IL-9 in the pathogenesis of RA, with a view to providing diagnostic and therapeutic strategies for the clinical treatment of RA and SNRA.
Material and methods
Clinic samples
Cases and healthy controls were retrospectively collected from January 2022 to December 2022 by reviewing electronic medical records. The included patients were assigned to 3 groups based on the study of clinical records. Ninety-nine patients were diagnosed with RA according to the American College of Rheumatology (ACR) 2010 classification criteria for RA, of which 10 patients were serologically tested as RF antibody-negative and ACPA antibody-positive, i.e., the RA + RF negative group, 12 patients were serologically tested as RF antibody-positive, ACPA antibody-negative, i.e., the RA + ACPA negative group, and 13 patients were serologically tested as RF antibody-negative, ACPA antibody-negative, i.e., the SNRA group. In addition, 4 healthy people, from the normal group, were included in the study. The researchers reviewed the medical records of all patients and collected relevant clinical and serologic data. Before the study, it received ethical approval from the local ethics committee and all participants provided their informed consent by signing a consent form.
Enzyme-linked immunosorbent assay (ELISA)
After collecting blood samples, they were subjected to centrifugation at 1,000 g for 10 min. The resulting serum was carefully separated and collected for further analysis. We measured the levels of serum markers using ELISA with a specialized commercial human IL-9 ELISA Kit (CSB-E04642h, Cusabio, Wuhan, China), human Gal1 ELISA Kit (CSB-EL012882h, Cusabio), human anti-CII ELISA Kit (HB3371-Hu, Shanghai Hengyuan Biotechnology Co., Ltd, Shanghai, China), human sSR-A ELISA Kit (HB3370-Hu, Shanghai Hengyuan Biotechnology Co., Ltd), human anti-Sa ELISA Kit (ml382511V), human RF ELISA Kit (ml060678V), human ACPA ELISA Kit (ml233305V), rat IL-9 ELISA Kit (CSB-E07294r, Cusabio), rat Gal1 ELISA Kit (JL25762, Shanghai Jianglai Biotechnology Co., Ltd, Shanghai, China), rat anti-CII ELISA Kit (HB1364-Ra, Shanghai Hengyuan Biotechnology Co., Ltd), rat sSR-A ELISA Kit (HB1363-Ra, Shanghai Hengyuan Biotechnology Co., Ltd), rat anti-Sa ELISA Kit (ml202241V), rat RF ELISA Kit (ml059541V), and rat ACPA ELISA Kit (ml201458V). The ELISA kits of human anti-Sa, human RF, human ACPA, rat anti-Sa, rat RF, and rat ACPA were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China. The experimental procedure was carried out according to the manufacturer’s instructions. The optical density (OD) of each well was measured at 450 nm using an enzyme marker (MB-530, HuiSong, Shenzhen, China).
Animal model and treatment
Male SD rats (8 weeks old, Hunan Slake Jinda Laboratory Animal Center, Changsha, China) were acclimatized and fed for 1 week, and a type II collagen (CII)-induced RA (CIA) model was established using a method previously reported in the literature [13]. Briefly, six rats were selected as the control group and the remaining six rats as the CIA group, and were initially immunized with complete Freund’s adjuvant (CFA)-emulsified CII. Approximately 200 µl of bovine CII emulsion (2.0 mg/ml) was injected subcutaneously into the tail root. Eight days after the initial immunization, the immunization was reinforced with 100 µl of CII emulsion (2.0 mg/ml) plus FIA. The control group was injected with an equal amount of saline. CII emulsion was prepared by using high purity CII (4 mg/ml, Sigma-Aldrich, St Louis, MO, USA) configured as a 2 mg/ml solution of 0.05 M/l glacial acetic acid, which was then mixed 1 : 1 by volume with 4 mg/ml CFA (HY-153808, MCE, NJ, USA) and emulsified using a homogenizer. To investigate the effect of IL-9 on arthritis in CIA rats, we randomly divided CIA rats into 3 groups: the CIA group (CIA rats treated with rat isotype IgG antibodies (100 mg/week, 31933, Invitrogen, Carlsbad, CA, USA)), the CIA + IL-9 group (CIA rats treated with recombinant rat IL-9 (rrIL-9, 200 ng/d, P6285, Beyotime, Shanghai, China)), and the CIA + IL-9 inhibitor group (CIA rats treated with anti-rat IL-9 neutralizing monoclonal antibodies (mAb; 100 mg/week; BioLegend, Beijing, China)), n = 6 each group. After 42 days of initial immunization, these animals were euthanized in a CO2 chamber to obtain the required blood and knee tissues.
Evaluation of arthritis severity
After the initial immunization, the body weights of rats were recorded once a week, and the severity of arthritis was scored. The severity of arthritis in the ankle joints was assessed using a scoring system, with scores ranging from 0 to 4. A score of 0 indicated no redness, while scores of 1, 2, 3, and 4 represented increasing levels of red spots, mild swelling, moderate swelling, severe swelling, and ankle deformity with difficulty in walking, respectively [14]. Additionally, the paw volume of the right hind limb was measured every week as an index of paw swelling. Macroscopic photographs were taken to observe the arthritic parts of the rats on the 42nd day. At the end of the animal experiment, the rat knee joint tissues were taken, and H&E staining was performed to observe the histological changes in the knee joint.
HE staining
Rat knee tissue was collected and fixed using 4% paraformaldehyde. After fixation, the tissues were embedded in paraffin. Tissue sections were then prepared and stained with hematoxylin and eosin. The stained sections were then sealed with neutral gum and examined under a light microscope (Olympus, Japan) for further observation.
Analysis of saffron O-solid green staining
We analyzed cartilage damage by performing senna O-solid green staining. Rat knee tissue sections were then prepared and stained with a solid green solution and saffron O solution. The stained sections were then sealed with neutral gum and examined under a light microscope (Olympus, Japan) for further observation.
Flow cytometry assay
Rat knee tissue was treated with 0.25% trypsin to obtain a cell suspension. The treated cells were then stained with the appropriate antibodies. CD4-APC (47-0041-82, eBioscience, CA, Santiago, USA) and IL17-PE (12-7177-81, eBioscience) were used to detect Th17 cells, and CD25-FITC (MA1-35144, eBioscience) and Foxp3-PE (12-5773-82, eBioscience) were used to detect Treg cells. The levels of Th17 and Treg cells were analyzed using a flow cytometer (a00-1-1102, Beckman, California, USA).
RT-qPCR
Total RNA was extracted from rat knee tissue using Trizol reagent (15596026, Thermo Fisher), and then the mRNA was reversed to cDNA using the mRNA Reverse Transcription Kit (CW2569, CWBIO, Beijing, China). 2 µl of cDNA was added into mix solution containing 15 µl of 2 × SYBGREEN PCR Master Mix (CW2601, CWBIO), 1 µl of each of the forward and reverse specific primers, and ddH2O to make up to 30 µl for RT-qPCR detection. The primer sequences used for RT-qPCR can be found in Table 1.
Immunofluorescence staining
Rat knee tissue sections were placed in sodium borohydride solution, stained with Sudan black staining solution, then blocked with 10% normal serum/5% BSA. The sections were incubated with appropriate primary antibodies: CD68 (AWA00050, 1 : 50, Abiowell, Changsha, China), CD206 (AWA44419, 1 : 50, Abiowell), iNOS (AWA45150, 1 : 50, Abiowell) overnight at 4oC, followed by incubation with CoraLite488-conjugated Affinipure Mouse Anti-Rabbit IgG (H + L) (SA00013-2, 1 : 500, Proteintech, Chicago, IL, USA) at 37°C for 60 min. DAPI was used to stain the nuclei.
Statistical analysis
All data were analyzed using SPSS 22.0 software, and the results were presented as mean ± standard deviation. The difference between the two groups was analyzed using the Student’s t-test. One-way ANOVA and two-way ANOVA were used for multiple group analysis. A result with p < 0.05 was considered statistically significant. Spearman correlation was used for analyzing correlations between serum markers in clinical samples of serum. Pearson correlation was used for analyzing the correlation between serum IL-9 and arthritis scores in CIA rats.
Results
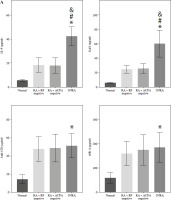
Detection of serum marker levels in RA patients with different antibody types
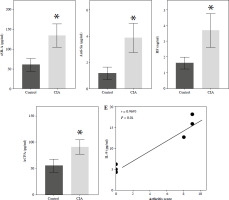
We determined the levels of the novel serum markers IL-9, Gal1, anti-CII, sSR-A, and anti-Sa, as well as the traditional markers RF and ACPA, in serum samples from normal, RA + RF-negative, RA + ACPA-negative, and SNRA patients, respectively. The demographic characteristics are detailed in Supplementary Table 1. The results showed that RF and ACPA levels in the SNRA group were not significantly different compared with the normal group, again validating that RF and ACPA are not able to diagnose all RA patients. The levels of the novel markers IL-9, Gal1, and anti-Sa were higher in the serum of the SNRA group than in other groups, especially the difference in IL-9, suggesting that IL-9 is helpful in the diagnosis of RA. In addition, the serum levels of anti-CII and sSR-A in the SNRA group were higher than those in the normal group but did not differ from those in the RA + RF-negative and RA + ACPA-negative groups (Fig. 1A). We also analyzed the correlation between the serum markers IL-9, Gal1, anti-CII, sSR-A, anti-Sa, RF, and ACPA in RA patients, and the results showed a positive correlation between these markers (Fig. 1B). Th17 cells are pathogenic T cells in the development of autoimmune inflammatory diseases, whereas Treg cells function to suppress excessive effector T-cell responses [15]. The balanced relationship between Treg cells and Th17 cells plays an important role in the development of RA. As shown in Supplementary Figure 1A, B, the level of Th17 in the RA group was significantly higher than that in the normal group, while the level of Treg and the ratio of Treg/Th17 were significantly lower than those in the normal group, with the most significant difference in the SNRA group. These findings indicate that the inflammatory response is amplified in RA, with SNRA exhibiting the most pronounced inflammatory reaction. Furthermore, we also detected the serum levels of the pro-inflammatory cytokine IL-17 and the anti-inflammatory cytokine IL-10. The results demonstrated that the levels of IL-17 were significantly higher and IL-10 significantly lower in the SNRA group than in the other groups (Supplementary Fig. 1C).
Fig. 1
Detection of serum marker levels in rheumatoid arthritis (RA) patients with different antibody types. A) ELISA for the determination of IL-9, Gal1, anti-CII, sSR-A, anti-Sa, RF, and ACPA levels in serum of clinical samples. *p < 0.05 vs. the normal group, #p < 0.05 vs. the RA + RF negative group, &p < 0.05 vs. the RA + ACPA negative group. B) Spearman’s analysis of the correlation of the above serum markers. The descriptive statistics are presented as mean ±SD. *p < 0.05, **p < 0.01, ****p < 0.0001, ns. represents no significance. n = 4 in normal group, n = 10 in RA + RF negative group, n = 12 in RA + ACPA negative group, n = 13 in SNRA group
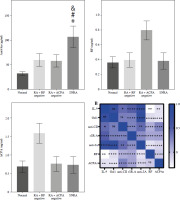
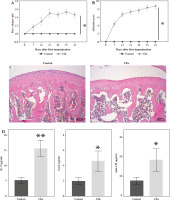
Validation of serum marker expression in CIA rats
To further explore the in vivo role of these serum markers, we established a CIA rat model. Throughout the experiment, we closely monitored the overall condition of the rats. The control rats appeared to be in good health, displaying normal movement and behavior. In contrast, the CIA rats exhibited progressive development of polyarthritis following CII-enhanced immunization. This was evident through various symptoms, such as weight loss and swelling in the foot joints (Supplementary Fig. 2A, B). To assess the severity of arthritis, we measured the foot volume and assigned an arthritis score, with larger paw volume and higher scores indicating a more severe disease. After establishing the CIA model, we observed a significant increase in paw volume and arthritis score in the CIA group compared to the control group (Fig. 2A, B). Histological analysis of joint tissues using HE staining further confirmed the presence of synovial hyperplasia, as well as cartilage and bone destruction in the CIA group (Fig. 2C). The above results indicated that the CIA model was successfully established. Next, we examined the serum levels of novel serum markers such as IL-9 and conventional markers of RF and ACPA in control and CIA rats, respectively, and the results showed that the levels of these markers were higher in the CIA group than in the control group, and among them, the level of IL-9 showed the most significant difference between the control and CIA groups (Fig. 2D). Further, we analyzed the correlation between IL-9 and arthritis scores, and the results showed that the levels of IL-9 were positively correlated with arthritis scores (Fig. 2E). Therefore, we hypothesized that IL-9 is the most sensitive serum marker for the diagnosis of RA.
Fig. 2
Validation of serum marker expression in CIA rats. A) Rat paw volume. B) Rat arthritis score. C) H&E staining was used to assess histopathologic damage in joints. D) ELISA for serum markers (IL-9, Gal1, anti-CII, sSR-A, anti-a, RF, ACPA) in rats. The descriptive statistics are presented as mean ±SD. *p < 0.05, **p < 0.01, n = 3 E) Pearson analysis of the correlation between IL-9 and arthritis scores. The descriptive statistics are presented as mean ±SD. *p < 0.05, **p < 0.01, n = 3
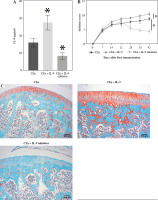
Interleukin 9 affects the severity of arthritis in CIA rats
To explore the potential role of IL-9 in the pathogenesis of RA, we applied IL-9 and IL-9 inhibitors to observe their effects on arthritis in CIA rats. First, we detected the level of IL-9 in the serum of CIA rats, which was significantly higher in the CIA + IL-9 group and lower in the CIA + IL-9 inhibitor group compared with the CIA group (Fig. 3A). By observing the arthritic sites of CIA rats, we found that paw swelling was more pronounced in rats in the CIA + IL-9 group compared to the CIA group, while paw swelling was significantly lower in rats in the CIA + IL-9 inhibitor group (Supplementary Fig. 2C). Arthritis scores also indicated that the degree of arthritis was higher in the rats in the CIA + IL-9 group compared to the CIA group, whereas the degree of arthritis was lower in the rats in the CIA + IL-9 inhibitor group (Fig. 3B). In addition, we analyzed the cartilage damage in rats by saffron O-solid green staining, which showed that the total cartilage erosion was significant in rats in the CIA group, and the bone damage was aggravated in the IL-9-treated group while it was reduced in the IL-9-inhibitor-treated group (Fig. 3C). The above results indicated that IL-9 aggravated the arthritis in CIA rats.
Targeted inhibition of IL-9 improves inflammation and Treg/Th17 immune balance in CIA rats
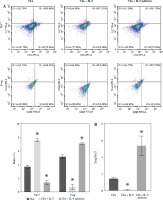
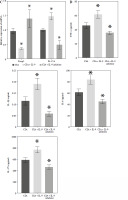
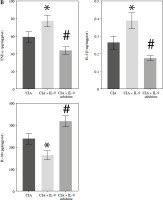
We further explored the potential mechanisms by which IL-9 affects RA. IL-9 has been shown to exert an impact on the functionality of both Th17 cells and Treg cells. It acts synergistically with transforming growth factor β (TGF-β) to facilitate the differentiation of naïve CD4+ T cells into TH17 cells. Additionally, IL-9 enhances the suppressive capabilities of FOXP3+ Treg cells. Consequently, IL-9 plays a critical role in modulating the balance between Treg and Th17 cell populations [16]. By employing flow cytometry, we evaluated the ratio of Treg to Th17 cell infiltration in the arthritic tissues of CIA rats. The findings revealed a notably higher abundance of Th17 cells and a concomitantly lower abundance of Treg cells in the CIA + IL-9 group in comparison to the CIA group. In other words, the ratio of Treg to Th17 cells was found to be diminished in the CIA + IL-9 group. The opposite trend was observed after treatment with IL-9 inhibitors (Fig. 4A, B). We further examined the mRNA levels of Foxp3, a marker for Treg, and IL-17A, a marker for Th17, and the results showed a consistent trend with the detection of Treg and Th17 cells (Fig. 4C). In addition, we examined the levels of the inflammatory cytokines tumor necrosis factor α (TNF-α), IL-1β, IL-6, and IL-17A in the synovial serum of CIA rats. It was observed that the levels of inflammatory cytokines were higher in the CIA + IL-9 group, while they were lower in the CIA + IL-9 inhibitor group compared to the CIA group. These results suggest that inhibition of IL-9 increases Treg/Th17 cells’ immune balance and improves inflammation in CIA rats.
Fig. 4
Targeted inhibition of IL-9 improves inflammation and Treg/Th17 immune balance in CIA rats. A) Proportion of Treg and Th17 cells infiltrated in arthritic tissues by flow cytometry assay. B) Change in Treg/Th17 ratio. C) RT-qPCR detection of foxp3 and IL-17A expression in arthritic tissues. D) Determination of TNF-α, IL-1β, IL-6, and IL-17A levels in the synovial serum of CIA rats by ELISA. The descriptive statistics are presented as mean ±SD. *p < 0.05 vs. the CIA group, n = 3
Targeted inhibition of IL-9 regulates M2 and M1 macrophage activation
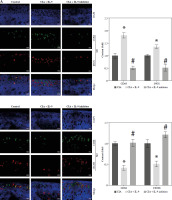
The development of RA is associated with an imbalance between pro-inflammatory M1 macrophages and anti-inflammatory M2 macrophages [17]. To investigate whether IL-9 could affect M1 and M2 macrophage activation, we examined the expression of M1 macrophage markers (CD68 and iNOS) and M2 macrophage markers (CD68 and CD206) in arthritic tissues, respectively. In the CIA + IL-9 group, the expression of the M1 macrophage markers was found to be higher, while the expression of the M2 macrophage markers was lower, compared to the CIA group. Conversely, treatment with the IL-9 inhibitor showed an opposite trend, with the expression of M1 macrophage markers lower and the expression of M2 macrophage markers higher (Fig. 5A). Additionally, we conducted further analysis to assess the levels of the pro-inflammatory cytokines TNF-α and IL-1β, as well as the anti-inflammatory cytokine IL-10, in the arthritic tissues. Our findings revealed that IL-9 administration increased the levels of pro-inflammatory cytokines and reduced the levels of anti-inflammatory cytokines in arthritic tissues of CIA rats. Conversely, treatment with the IL-9 inhibitor exhibited the opposite effect, with decreased levels of pro-inflammatory cytokines and increased levels of anti-inflammatory cytokines (Fig. 5B). These results suggest that inhibition of IL-9 modulates M2 and M1 macrophage activation, and alleviates tissue inflammation.
Fig. 5
Targeted inhibition of IL-9 regulates M2 and M1 macrophage activation. A) Immunohistochemical detection of M1 (CD68, iNOS) and M2 (CD68, CD206) macrophage expression in arthritic tissues B) Detection of TNF-α, IL-1β, IL-10 levels in arthritic tissues by ELISA. The descriptive statistics are presented as mean ±SD. *p < 0.05 vs. the CIA group, n = 3
Discussion
Rheumatoid arthritis is a polyarticular inflammatory autoimmune joint disease, the main clinical manifestations of which are musculoskeletal pain, swelling, and stiffness, which severely impairs patients’ physical function and quality of life [18]. Currently, the clinical diagnosis of RA relies on clinical symptoms, imaging examinations, and laboratory biomarkers for differential diagnosis. Among them, the detection of serum autoantibodies in laboratory tests is an important auxiliary means for the diagnosis of RA. However, a proportion of RA patients are negative for two major types of autoantibodies – RF and ACPA – in serologic-related tests, i.e., SNRA [6, 7]. Therefore, new diagnostic biomarkers are needed to complement the existing ones. We selected IL-9, Gal1, anti-CII, sSR-A, and anti-Sa as possible serum markers of RA based on previous reports in the literature [19-23] and validated them by testing clinical serum samples from RA patients and serum samples from CIA model rats, and found that the serum levels of IL-9 were significantly higher in the serum of SNRA patients and CIA model rats than in other groups. These results indicate that IL-9 has potential as a novel biomarker for the diagnosis of RA.
Interleukin 9 is a cytokine derived from Th9 cells [24]. It affects both Th17 and Treg cell functions [25, 26], synergizes with TGF-β to induce the differentiation of native CD4+ T cells to Th17 cells, and increases the inhibitory function of FOXP3+ Treg cells [25], suggesting its dual function in inflammatory diseases [27]. This role has been found in RA. This study suggests that IL-9 promotes neutrophil survival, function, and differentiation of Th17 cells in RA, and that inhibition of IL-9 can affect the production of pathogenic cytokines in RA, leading to better disease control [11]. In addition, it has been shown that IL-9 can be induced by Th9- and Th17-stimulating conditions in RA and that differentiation of Th9 cells may also be induced by Th17-stimulating conditions, emphasizing the joint role of Th9- and Th17 in RA [28]. Our findings showed that inhibition of IL-9 significantly reduced the degree of arthritis in rats by both arthritis scoring and analysis of cartilage damage, and the flow cytometry assay and RT-qPCR results also showed that inhibition of IL-9 decreased the content of Th17 cells and increased the content of Treg cells. However, in a study by Rauber et al., IL-9 deficiency in an antigen-induced arthritis (AIA) mouse model slowed down the proliferation of type 2 innate lymphoid cells (ILC2) and promoted the activation of Treg cells, leading to chronic arthritis, excessive cartilage destruction, and bone turnover [29]. This may be due to the differences in the physiopathological conditions that lead to different roles of IL-9 in different RA inflammation models.
Although the immunopathophysiological mechanisms of rheumatoid arthritis have not been fully elucidated, it is widely recognized that inappropriate interactions between macrophages and T cells play a significant role. Activation of M1 or M2 macrophages can influence the development of Th1 or Th2 responses, respectively [30]. In patients with RA, there is activation of the M1/Th1 response in an inflammatory environment characterized by Toll-like receptor (TLR) and interferon (IFN) signaling. This activation leads to the production of pro-inflammatory cytokines such as TNF-α, IL-1, IL-12, IL-18, and IFN-γ, as well as chemokines and matrix metalloproteinases. This inflammatory milieu contributes to the development of osteoclastogenesis, erosion, and progressive joint destruction. On the other hand, activation of the M2/Th2 response results in the release of growth factors and anti-inflammatory cytokines including IL-4, IL-10, IL-13, and TGF-β. These factors are involved in promoting an anti-inflammatory state and may contribute to clinical remission in RA [17]. Nevertheless, the effect of IL-9 on macrophage polarization in RA has rarely been reported. Our study demonstrated that inhibition of IL-9 inhibited the activation of M1 macrophages and promoted the activation of M2 macrophages, thereby decreasing the levels of pro-inflammatory cytokines TNF-α and IL-1β, and elevating the level of the anti-inflammatory cytokine IL-10.
However, it is crucial to acknowledge and address the limitations and drawbacks of this study. Firstly, while our findings suggest that IL-9 holds promise as a diagnostic marker for SNRA, further comprehensive studies are needed to validate this result. Additionally, it is imperative to expand the sample size and include a larger number of patients with SNRA in future investigations. Secondly, the study lacks an in vitro component in human RA. This would provide a more comprehensive understanding of the role of IL-9 in the pathogenesis of RA.
Conclusions
In conclusion, our results suggest that IL-9 has potential as a diagnostic marker for SNRA. Moreover, inhibition of IL-9 attenuated the severity of arthritis in CIA rats by improving inflammation and Treg/Th17 immune homeostasis and modulating M2 and M1 macrophage activation. Therefore, IL-9 may become a new serum marker for the diagnosis of SNRA and may provide a new target for the treatment of RA.